Contact Us
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.
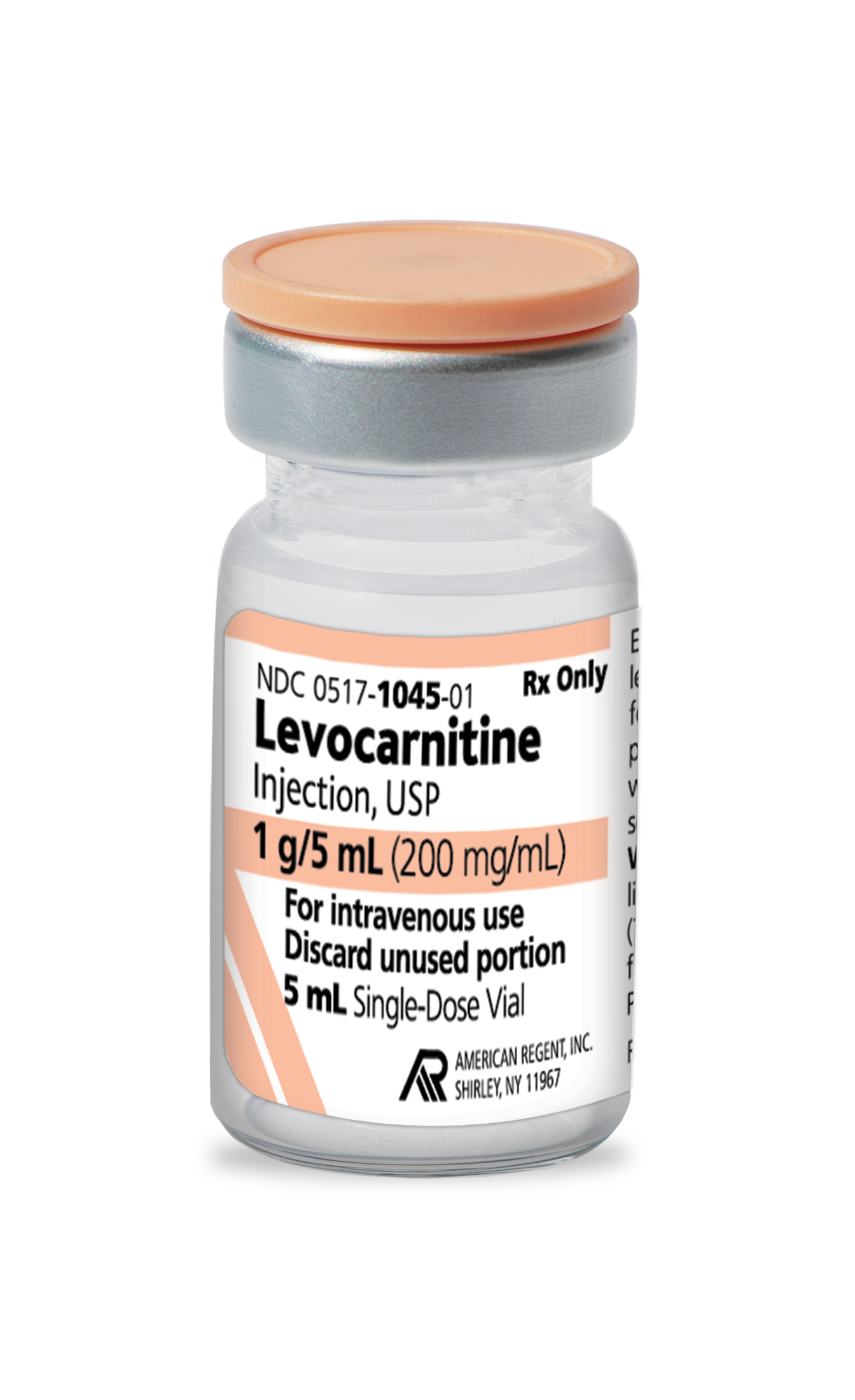
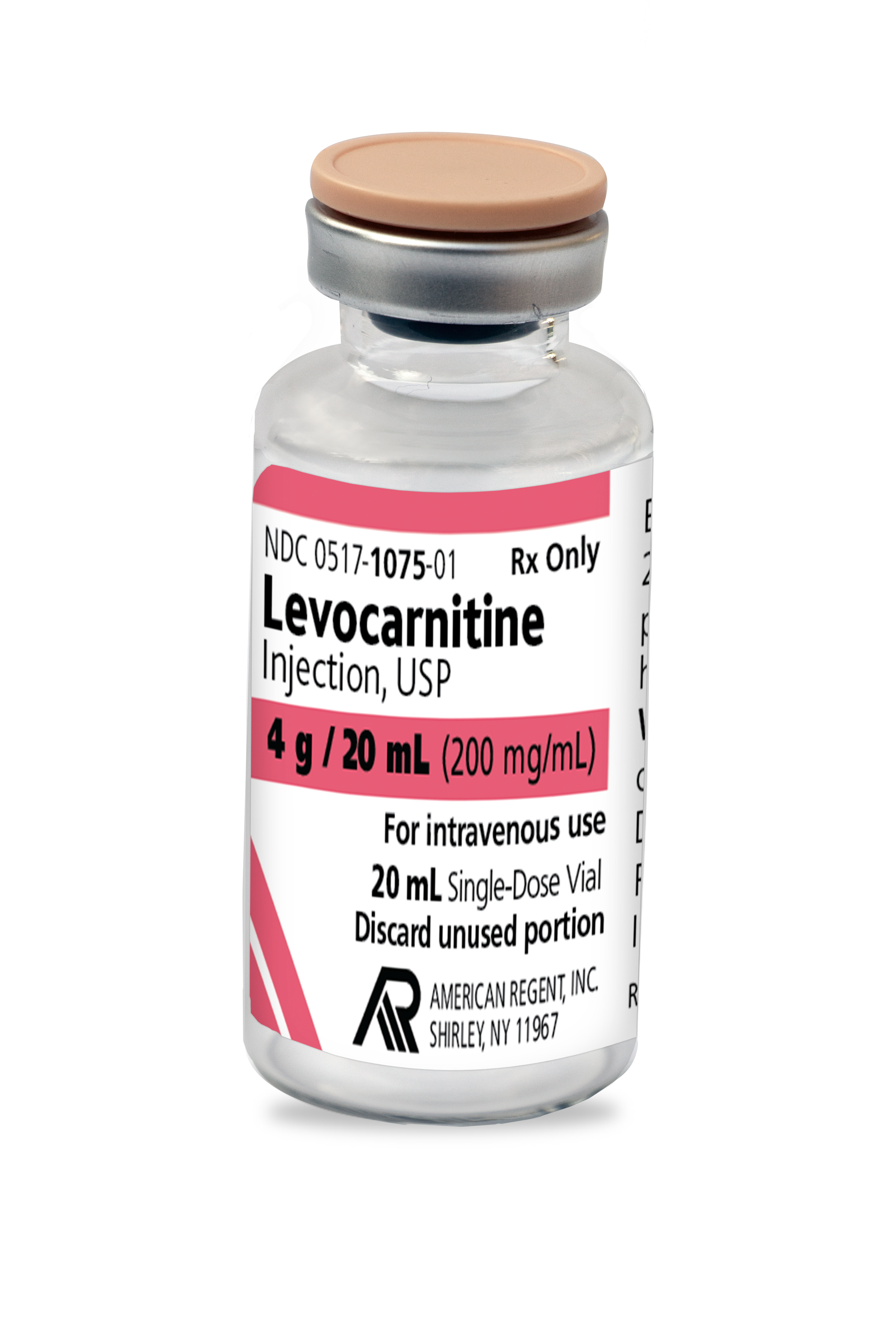
FOR INTRAVENOUS USE ONLY
INDICATIONS AND USAGE
For the acute and chronic treatment of patients with an inborn error of metabolism which results in secondary carnitine deficiency.
For the prevention and treatment of carnitine deficiency in patients with end-stage renal disease who are undergoing dialysis.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
None known.
WARNINGS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, laryngeal edema, and bronchospasm have been reported following levocarnitine administration, mostly in patients with end-stage renal disease who are undergoing dialysis. Some reactions occurred within minutes after intravenous administration of levocarnitine.
If a severe hypersensitivity reaction occurs, discontinue levocarnitine treatment and initiate appropriate medical treatment. Consider the risks and benefits of re-administering levocarnitine to individual patients following a severe reaction. If the decision is made to re-administer the product, monitor patients for a reoccurrence of signs and symptoms of a severe hypersensitivity reaction.
PRECAUTIONS
General
The safety and efficacy of oral levocarnitine has not been evaluated in patients with renal insufficiency. Chronic administration of high doses of oral levocarnitine in patients with severely compromised renal function or in ESRD patients on dialysis may result in accumulation of the potentially toxic metabolites, trimethylamine and trimethylamine-N-oxide, since these metabolites are normally excreted in the urine.
Drug Interactions
Reports of INR increase with the use of warfarin have been observed. It is recommended that INR levels be monitored in patients on warfarin therapy after the initiation of treatment with levocarnitine or after dose adjustments.
Pregnancy
There are no adequate and well-controlled studies in pregnant women. This drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Levocarnitine supplementation in nursing mothers has not been specifically studied. Studies in dairy cows indicate that the concentration of levocarnitine in milk is increased following exogenous administration of levocarnitine. In nursing mothers receiving levocarnitine, any risks to the child of excess carnitine intake need to be weighed against the benefits of levocarnitine supplementation to the mother. Consideration may be given to discontinuation of nursing or of levocarnitine treatment.
ADVERSE REACTIONS
Clinical Trials Experience
Transient nausea and vomiting have been observed. Less frequent adverse reactions are body odor, nausea, and gastritis.
Postmarketing Experience
The following adverse reactions have been reported:
Neurologic Reactions: Seizures have been reported to occur in patients, with or without pre-existing seizure activity, receiving either oral or intravenous levocarnitine. In patients with pre-existing seizure activity, an increase in seizure frequency and/or severity has been reported.
Hypersensitivity reactions: Anaphylaxis, laryngeal edema, and bronchospasm.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report adverse drug events to American Regent, Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
REF-1593 2/2022